The illicit drug supply in America today is as dangerous as it has ever been. Fentanyl, badly mixed into $4 bags, cross contaminated into cocaine or hidden in counterfeit pills, is poisoning our people,
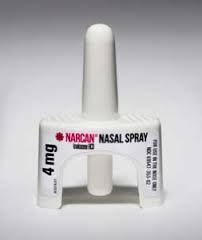
This week the Federal Food and Drug Administration (FDA) approved a Naloxone nasal spray for over-the-counter (OTC) sales. This is a tremendous step toward universal availability of the life-saving anti-opioid drug.
But before we celebrate there are three challenges ahead.
OTC naloxone must be affordable, accessible, and available in sufficient quantity to meet the needs of the public, harm reduction and emergency medical services.
Affordability. Currently Naloxone for those with insurance is free or comes with a small co-pay. If naloxone is sold over the counter at its current price point, and if it is no longer covered by insurance, it will be unaffordable to many who need it most.
Accessibility. If Naloxone is only hidden behind pharmacist and store counters, and not on the common shelfs next to the aspirin and cold medicines, it may not be obtained by those who fear being stigmatized.
Availability. If Naloxone is not produced in sufficient quantities to stock both store shelves and the emergency in-bags of our first responders as well as the backpacks of those who the front lines as harm reductionists, the overdose poisoning crisis will not get better.
Naloxone is a key weapon to prevent poisoning deaths and keep people alive until we can get them to a safe place in their journey.
Affordability, Accessibility and Availability. We can do this.